ANTIBIOTIC STEWARDSHIP TOOLKIT
FOR DENTAL PROVIDERS
NOVEMBER 2017
Printed by Authority of the State of Illinois
P.O. # 273029 350 11/2017

1
Antibiotics Stewardship Toolkit for Dental Providers
The purpose of this toolkit is to provide Illinois dentists with resources to support appropriate antibiotic
prescribing as part of the Illinois Precious Drugs & Scary Bugs Campaign. The campaign aims to promote the
judicious use of antibiotics in the outpatient setting. Antibiotic resistance is among the greatest public health
threats today, leading to 2 million infections and 23,000 deaths each year
1
. In community settings in the
United States, dentists are the fourth highest prescribers of antibiotics and have an important role to play to
ensure that antibiotics are prescribed only:
when needed;
at the right dose;
for the right duration; and
at the right time.
2
The Centers for Disease Control and Prevention (CDC) recommends that all outpatient health care providers,
including dentists, take steps to measure and improve how antibiotics are prescribed using the Core Elements
of Outpatient Antibiotic Stewardship as a framework. The four core elements include:
Commitment: Demonstrate dedication to optimizing antibiotic prescribing and patient safety
Action for Policy and Practice: Implement a practice change to improve antibiotic prescribing
Tracking and Reporting: Monitor antibiotic prescribing practices
Education and Expertise: Provide educational resources to health care providers and patients
This toolkit is organized around these core elements and includes provider and patient resources. It is
intended to be used as a practical action planning guide. For more information please visit
www.cdc.com/antibiotic-use or e-mail DPH.DPSQ@Illinois.gov.
Funding for this toolkit was made possible by the Centers for Disease Control and Prevention. The views expressed in
this document do not necessarily reflect the official policies of the US Department of Health and Human Services, nor
does the mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government.
1
Centers for Disease Control and Prevention. (2017). Antibiotic/Antimicrobial Resistance. Available at:
https://www.cdc.gov/drugresistance/index.html
2
Roberts, et. al. (2013). Antibiotic prescribing by general dentists in the United States. Available at:
http://jada.ada.org/article/S0002-8177(16)30942-4/fulltext

2
Contents Page
INTRODUCTION
Gubernatorial Proclamation……………………………………………………………………………………………….…………..…………..….…3
The Need…………………………………………………………………………………………………………………………………………..……..…..…..4
What YOU Can Do: Core Elements of Outpatient Antibiotic Stewardship……………………………………………………..…..5
1. MAKE A COMMITMENT………………………………………………………………………………………………………………………..….….….6
You can demonstrate commitment to optimizing antibiotic prescribing and patient safety by:
Submitting a letter of commitment to IDPH
Displaying a customizable commitment poster
2. ACT
Use evidence-based diagnostic criteria and treatment recommendations to improve
antibiotic prescribing with the resources provided.
Evidence-based Practices
Checklist for Antibiotic Prescribing in Dentistry …………………………………………………………………………….….……..10
Combating Antibiotic Resistance ……………………………………………………………………………………………………..………11
Antibiotic Prophylaxis Update 2017 ……………………………………………………………………………………………………..….15
Treatment Guidelines
Use of Antibiotic Therapy for Pediatric Patients ……………………………………………………………………………………...18
Management of Patients with Prosthetic Joints – Chairside Guideline ………………………………………………….…21
Nonsurgical Treatment of Chronic Periodontitis by Scaling and Root Planing……………………………………..……22
3. TRACK AND REPORT
Implement at least one system to track and report antibiotic prescribing. Page 24 includes resources for
outcome tracking and continuing medical education. Please complete a self-evaluation of your prescribing
practices by January 5, 2018 using the survey provided.
Illinois Dental Provider Survey
4. EDUCATE
Educate patients about appropriate antibiotic use and the potential harms of antibiotic
treatment with these resources:
Antibiotic Safety : Do’s & Don’ts at the Dentist…………………………………………………………………………….………….26
What is Antibiotic Prophylaxis? ....................................................................................................................27
What is Infective Endocarditis?.....................................................................................................................28
Improving Antibiotic Use …………………………………………………………………………………………………………………....…..30
REFERENCES……………………………………………………………………………………………………………………………………..….………….…..32

1
l 7 it·Sl -/
SECRETARY OF STATE
GOVERNOR
3
4
The Need
Antibiotic Prescribing in Outpatient Settings in the United States
Over 60% of all antibiotic expenditures are associated with the outpatient setting.
At least 30% of antibiotics prescribed in the outpatient setting are unnecessary.
3
Antibiotic Prescribing Among Dentists in the United States
Dentists account for 10% of outpatient antibiotic prescriptions, or 24.5 million prescriptions. In 2013,
dentists wrote an average of 205 antibiotic prescriptions each.
Overall, in the United States dentists prescribe 77.5 prescriptions per 1,000 people.
Illinois dentists prescribe, on average, 79.6 prescriptions per 1,000 people (higher than the national
average).
Among dentists, the three highest prescribed types of antibiotics are penicillin (69.6%), lincosamides
(14.6%), and macrolides (5.4%).
4
Unintended Consequences of Antibiotic use
Adverse events from antibiotics include rashes, diarrhea, and severe allergic reactions. These lead to an
average of 143,000 emergency department visits each year and contribute to excess health care costs.
5
Antibiotic treatment is the most important risk factor for Clostridium difficile infection, which can cause
life-threatening diarrhea. A 2013 study found that over 40% of patients with C. difficile infection visited
a dentist or physician’s office in the preceding four months.
6
3
Centers for Disease Control and Prevention: https://www.cdc.gov/antibiotic-use/community/programs-measurement/measuring-antibiotic-
prescribing.html
4
Roberts, R., Bartoces, M., Thompson, S. and Hicks, L. (2017). Antibiotic prescribing by general dentists in the United States, 2013. The Journal of the
American Dental Association, 148(3), pp.172-178.e1.
5
Centers for Disease Control and Prevention: https://www.cdc.gov/medicationsafety/program_focus_activities.html
6
Roberts, R., Bartoces, M., Thompson, S. and Hicks, L. (2017). Antibiotic prescribing by general dentists in the United States, 2013. The Journal of the
American Dental Association, 148(3), pp.172-178.e1.

6
1. MAKE A COMMITMENT
A commitment from your dental office to prescribe antibiotics appropriately and
engage in antibiotic stewardship is critical to improving antibiotic prescribing.
Here are some ways your dental office can demonstrate commitment:
Submit the enclosed statement of commitment to the Illinois Department of
Public Health (IDPH). Providers making a commitment can choose to be
recognized on IDPH’s Website at www.tinyurl.com/drugsandbugs.
Display public commitment to antibiotic stewardship in your office (see sample
templates on page 7).
Include antibiotic stewardship-related duties in position descriptions or job
evaluation criteria.
Educate all staff members on how to manage patient expectations about
appropriate antibiotic use.
9
2. Act
Dentists can implement policies and interventions to promote appropriate antibiotic
prescribing.
Use evidence-based diagnostic criteria and treatment recommendations
Evidence Based Practices
Checklist for Antibiotic Prescribing in Dentistry (page 10)
o Download here: http://tinyurl.com/dentalabxlist
Combating Antibiotic Resistance (page 11)
Antibiotic Prophylaxis Update 2017 (page 15)
Treatment Guidelines
Guideline on the use of Antibiotic Therapy for Pediatric Patients (page 18)
Management of Patients with Prosthetic Joints – Chairside Guide (page 21)
Nonsurgical Treatment of Chronic Periodontitis by Scaling and Root Planing
with or without Adjuncts: Clinical Practice Guideline (page 22)
Review communications skills training for clinicians
Drexel University College of Medicine Physician Communication Modules:
interactive modules designed to enhance physician and patient
communication and address patient attitudes and beliefs that more care is
better care.
o Link to modules: http://tinyurl.com/cwmodules
CS267105-1
National Center for Emerging and Zoonotic Infectious Diseases
Division of Healthcare Quality Promotion
Checklist for Antibiotic Prescribing in Dentistry
Pretreatment
Correctly diagnose an oral bacterial infection.
Consider therapeutic management interventions, which may be sufficient to
control a localized oral bacterial infection.
Weigh potential benefits and risks (i.e., toxicity, allergy, adverse effects,
Clostridium difficile infection) of antibiotics before prescribing.
Prescribe antibiotics only for patients of record and only for bacterial infections
you have been trained to treat. Do not prescribe antibiotics for oral viral
infections, fungal infections, or ulcerations related to trauma or aphthae.
Implement national antibiotic prophylaxis recommendations for the medical
concerns for which guidelines exist (e.g., cardiac defects).
Assess patients’ medical history and conditions, pregnancy status, drug
allergies, and potential for drug-drug interactions and adverse events, any of
which may impact antibiotic selection.
Prescribing
Ensure evidence-based antibiotic references are readily available during
patient visits. Avoid prescribing based on non-evidence-based historical
practices, patient demand, convenience, or pressure from colleagues.
Make and document the diagnosis, treatment steps, and rationale for antibiotic
use (if prescribed) in the patient chart.
Prescribe only when clinical signs and symptoms of a bacterial infection
suggest systemic immune response, such as fever or malaise along with local
oral swelling.
Revise empiric antibiotic regimens on the basis of patient progress and, if
needed, culture results.
Use the most targeted (narrow-spectrum) antibiotic for the shortest duration
possible (2-3 days after the clinical signs and symptoms subside) for otherwise
healthy patients.
Discuss antibiotic use and prescribing protocols with referring specialists.
Patient Education
Educate your patients to take antibiotics exactly as prescribed, take antibiotics
prescribed only for them, and not to save antibiotics for future illness.
Staff Education
Ensure staff members are trained in order to improve the probability of patient
adherence to antibiotic prescriptions.
https://www.cdc.gov/antibiotic-use/community/downloads/dental-fact-sheet-FINAL.pdf
10
484 JADA, Vol. 135, April 2004
J
A
D
A
C
O
N
T
I
N
U
I
N
G
E
D
U
C
A
T
I
O
N
✷
✷
®
ASSOCIATION REPORT
A
R
T
I
C
L
E
1
Background. The ADA Council on Sci-
entific Affairs developed this report to pro-
vide dental professionals with current infor-
mation on antibiotic resistance and related
considerations about the clinical use of
antibiotics that are unique to the practice of
dentistry.
Overview. This report addresses the
association between the overuse of antibi-
otics and the development of resistant bac-
teria. The Council also presents a set of
clinical guidelines that urges dentists to
consider using narrow-spectrum antibacte-
rial drugs in simple infections to minimize
disturbance of the normal microflora, and to
preserve the use of broad-spectrum drugs
for more complex infections.
Conclusions and Practice
Implications. The Council recommends
the prudent and appropriate use of antibac-
terial drugs to prolong their efficacy and
promotes reserving their use for the man-
agement of active infectious disease and the
prevention of hematogenously spread infec-
tion, such as infective endocarditis or total
joint infection, in high-risk patients.
Combating antibiotic
resistance
ADA COUNCIL ON SCIENTIFIC AFFAIRS
F
or the past 70 years, antibiotic therapy has
been a mainstay in the treatment of bacterial
infectious diseases. However, widespread use
of these drugs by the health professions and
the livestock industry has resulted in an
alarming increase in the prevalence of drug-resistant
bacterial infections.
Worldwide, many strains of Staphylococcus aureus
exhibit resistance to all medically important antibacte-
rial drugs, including vancomycin,
1,2
and methicillin-
resistant S. aureus is one of the most frequent nosoco-
mial pathogens.
3
In the United States,
the proportion of Streptococcus pneumo-
niae isolates with clinically significant
reductions in susceptibility to β lactam
antimicrobial agents has increased
more than threefold.
4,5
Even more
alarming is the rate at which bacteria
develop resistance; microorganisms
exhibiting resistance to new drugs often
are isolated soon after the drugs have
been introduced.
6
This growing problem
has contributed significantly to the mor-
bidity and mortality of infectious dis-
eases, with death rates for communi-
cable diseases such as tuberculosis
rising again.
7,8
Disease etiologies also are changing.
In recent studies, staphylococci, particu-
larly S. aureus, have surpassed viridans
streptococci as the most common cause
of infective endocarditis.
9
Resistance
among bacteria of the oral microflora is
increasing as well. During the past decade, retrospec-
tive analyses of clinical isolates have clearly docu-
mented an increase in resistance in the viridans strep-
Any perceived
potential
benefit of
antibiotic
prophylaxis
must be
weighed
against the
known risks of
antibiotic
toxicity, allergy
and the
development,
selection and
transmission
of microbial
resistance.
tococci.
10
Further, strains of virtually
every oral microorganism tested exhibit
varying degrees of resistance to various
antibacterial agents.
11
This increase in antibacterial resis-
tance has been attributed primarily to
two different processes. First, reduced
susceptibility may develop via genetic
mutations that spontaneously confer a
newly resistant phenotype.
12
Alterna-
tively, the exchange of resistant deter-
minants between sensitive and resis-
tant microorganisms (of the same or
different species) may occur.
13
Regard-
less of the genetic basis of resistance,
the selective pressure exerted by
widespread use of antibacterial drugs is
the driving force behind this public
health problem. It is only through the
prudent and appropriate use of antibac-
terial drugs that their efficacy may be
prolonged.
Antibacterial drugs should be
ABSTRACT
Copyright ©2004 American Dental Association. All rights reserved.
11
Available online: http://jada.ada.org/article/S0002-8177(14)61234-4/pdf
JADA, Vol. 135, April 2004 485
ASSOCIATION REPORT
(1) make an accu-
rate diagnosis;
(2) use appropriate
antibiotics and dosing
schedules;
(3) consider using
narrow-spectrum
antibacterial drugs
(Table 1) in simple
infections to minimize
disturbance of the
normal microflora, and
preserve the use of
broad-spectrum drugs
(Table 2) for more com-
plex infections
17
;
(4) avoid unneces-
sary use of antibacte-
rial drugs in treating
viral infections;
(5) if treating empiri-
cally, revise treatment
regimen based on
patient progress or test
results;
(6) obtain thorough
knowledge of the side
effects and drug inter-
actions of an antibacte-
rial drug before pre-
scribing it;
(7) educate the
patient regarding
proper use of the drug
and stress the importance of completing the full
course of therapy (that is, taking all doses for the
prescribed treatment time).
Furthermore, the diagnosis and antibiotic
selection should be based on a thorough history
(medical and dental) to reveal or avoid adverse
reactions, such as allergies and drug interactions.
Any perceived potential benefit of antibiotic pro-
phylaxis must be weighed against the known
risks of antibiotic toxicity, allergy and the devel-
opment, selection and transmission of microbial
resistance.
15
It remains incumbent on dental practitioners,
as health care providers, to use antibacterial
drugs in a prudent and appropriate manner.
Adherence to the principles outlined here will aid
in extending the efficacy of the antibacterial
drugs that form the treatment foundation for
many infectious diseases. ■
reserved for the management of active infectious
disease and considered for the prevention of
hematogenously spread infection, such as infec-
tive endocarditis or total joint infection, in high-
risk patients (as defined by the American Heart
Association
14
and the American Dental Associa-
tion and the American Academy of Orthopedic
Surgeons
15
). One example of their use in man-
aging infectious disease is in the treatment of
aggressive periodontal disease, which use has
become well-accepted for optimal control of the
disease process.
16
The Council encourages further
research on the appropriate use of antibacterial
therapy in the management of oral diseases.
GUIDELINES FOR PRESCRIBING
ANTIBIOTICS
The following guidelines should be observed when
prescribing antibacterial drugs:
TABLE 1
NARROW-SPECTRUM* ANTIMICROBIAL AGENTS
ENCOUNTERED IN DENTISTRY.
†
GENERIC NAME CHARACTERISTICS
‡
COMMON INDICATIONS
FOR USE
Indicated for the
treatment of infections
caused by susceptible
microorganisms; used as a
prophylactic antibiotic in
high-risk patients allergic
to penicillin for the
prevention of both
bacterial endocarditis and
infections of total joint
replacements
Has been used as adjunct
in treatment of periodon-
titis and acute necrotizing
ulcerative gingivitis;
commonly coprescribed
with amoxicillin (Note: its
combined use with amoxi-
cillin or amoxicillin/
clavulanic acid has not
been approved by the U.S.
Food and Drug Adminis-
tration)
Use is limited to treatment
of minor infections such as
ulcerative gingivostom-
atitis, and to the prophy-
laxis and continued treat-
ment of streptococcal
infections
Clindamycin
Metronidazole
Penicillin V
Potassium
Bacteriostatic (bactericidal
at higher doses); active
against some aerobic gram-
positive cocci (including
Staphylococcus aureus, S.
epidermidis, streptococci and
pneumococci), some anaerobic
gram-negative bacilli, many
anaerobic gram-positive
non–spore-forming bacilli,
many anaerobic gram-positive
cocci and clostridia
Bactericidal; active against
most anaerobic cocci and both
gram-negative bacilli and
gram-positive spore-forming
bacilli
Bactericidal; cell-wall syn-
thesis inhibitor that is active
primarily against gram-
positive cocci (including
S. aureus), gram-positive and
gram-negative bacilli, and
spirochetes
* Active against a small number of organisms.
† Adapted in part from Ciancio.
17
‡ Bactericidal drugs directly kill an infecting organism; bacteriostatic drugs inhibit the proliferation of
bacteria by interfering with an essential metabolic process.
Copyright ©2004 American Dental Association. All rights reserved.
12
486 JADA, Vol. 135, April 2004
ASSOCIATION REPORT
TABLE 2
BROAD-SPECTRUM* ANTIMICROBIAL AGENTS ENCOUNTERED IN DENTISTRY.
†
GENERIC NAME CHARACTERISTICS
‡
COMMON INDICATIONS FOR USE
Commonly used as an empirical antibiotic for oral infec-
tions, sinusitis and skin infections; used as a prophylactic
antibiotic in high-risk patients for the prevention of bacte-
rial endocarditis and infections of total joint replacements
Used for the treatment of sinus, oral and respiratory
infections
Commonly used as an empirical antibiotic for oral infec-
tions, sinusitis and skin infections; used as a prophylactic
antibiotic in high-risk patients unable to take oral
medication for the prevention of both bacterial endocarditis
and total joint infections
Indicated for the treatment of infections caused by
susceptible microorganisms; used as a prophylactic
antibiotic in high-risk patients for the prevention of bacte-
rial endocarditis and infections of total joint replacements;
caution should be exercised when prescribing
cephalosporins for patients sensitive to penicillin
§
Used for the treatment of respiratory, urinary tract, skin
and biliary infections and for the treatment of septicemia
and endocarditis; used as a prophylactic antibiotic in high-
risk patients who are unable to take oral medications for
the prevention of both bacterial endocarditis and infections
of total joint replacements; caution should be exercised
when prescribing cephalosporins for patients sensitive to
penicillin
§
Indicated for the treatment of infections caused by
susceptible microorganisms; used as a prophylactic
antibiotic in high-risk patients for the prevention of bacte-
rial endocarditis and infections of total joint replacements;
caution should be exercised when prescribing
cephalosporins for patients sensitive to penicillin
§
Used as a prophylactic antibiotic in high-risk patients for the
prevention of bacterial endocarditis and infections of total
joint replacements; caution should be exercised when pre-
scribing cephalosporins for patients sensitive to penicillin
§
Indicated for the treatment of mild-to-moderate infections
caused by susceptible microorganisms; used as a
prophylactic antibiotic in high-risk patients allergic to
penicillin for the prevention of bacterial endocarditis
Indicated for the treatment of mild-to-moderate infections
caused by susceptible microorganisms; used as a prophy-
lactic antibiotic in high-risk patients allergic to penicillin
for the prevention of bacterial endocarditis
Indicated for the treatment of infections of upper and
lower respiratory tract, skin and soft-tissue infections of
mild-to-moderate severity; alternative to penicillin G and
other penicillins for treatment of gram-positive coccoid infec-
tions in patients with hypersensitivity to penicillins; used as
a prophylactic antibiotic in high-risk patients allergic to
penicillin for the prevention of bacterial endocarditis
Indicated for the treatment of periodontitis and acute
necrotizing ulcerative gingivitis (Note: to avoid the
gastrointestinal side effects of oral tetracyclines, localized
delivery systems of doxycycline and minocycline are
marketed for the treatment of periodontitis)
Amoxicillin
(Semisynthetic
Penicillin)
Amoxicillin
With
Clavulanic Acid
Ampicillin
(Semisynthetic
Penicillin)
Cefadroxil
(First-
Generation
Cephalosporin)
Cefazolin
(First-
Generation
Cephalosporin)
Cephalexin
(First-
Generation
Cephalosporin)
Cephradine
(First-
Generation
Cephalosporin)
Azithromycin
(Macrolide)
Clarithromycin
(Macrolide)
Erythromycin
(Macrolide)
Tetracycline
(Doxycycline,
Minocycline)
Bactericidal; active against many
gram-negative and gram-positive
organisms; not effective against
β-lactamase–producing bacteria
Bactericidal; active against a wide
spectrum of gram-negative and
gram-positive organisms, including
β-lactamase–producing bacteria
Bactericidal; active against many
gram-negative and gram-positive
organisms; not effective against
β-lactamase–producing bacteria
Bactericidal; active against
β-hemolytic streptococci,
staphylococci, Streptococcus pneumo-
niae, Escherichia coli, Proteus
mirabilis, Klebsiella and Moraxella
Bactericidal; active against group A
β-hemolytic streptococci,
Haemophilus influenzae, S. pneumo-
niae, E. coli, Enterobacter aerogenes,
P. mirabilis and Klebsiella
Bactericidal; active against β-
hemolytic streptococci, staphylococci,
S. pneumoniae, E. coli, P. mirabilis,
Klebsiella and Moraxella
Bactericidal; active against group A
β-hemolytic streptococci, H. influenza,
S. pneumoniae, E. coli, E. aerogenes,
P. mirabilis and Klebsiella
Bactericidal; active against a wide
range of aerobic gram-negative and
gram-positive organisms
Bactericidal; active against a wide
spectrum of aerobic and anaerobic
gram-positive and gram-negative
organisms
Bacteriostatic; active against
gram-positive bacteria, particularly
gram-positive cocci; provides limited
activity against gram-negative
bacteria
Bacteriostatic; active against
gram-positive and gram-negative
bacteria, mycoplasmas, rickettsial
and chlamydial infections
* Used as empirical antibiotics or when culture and sensitivity testing are not available.
† Adapted in part from Ciancio.
17
‡ Bactericidal drugs directly kill an infecting organism; bacteriostatic drugs inhibit the proliferation of bacteria by interfering with an
essential metabolic process.
§ Cross-hypersensitivity has been documented and will occur in up to 10 percent of patients who have a history of penicillin allergy.
18
Copyright ©2004 American Dental Association. All rights reserved.
13

JADA, Vol. 135, April 2004 487
ASSOCIATION REPORT
Address reprint requests to ADA Council on Scientific Affairs, 211 E.
Chicago Ave., Chicago, Ill. 60611.
1. Smith TL, Pearson ML, Wilcox KR, et al. Emergence of
vancomycin resistance in Staphylococcus aureus. Glycopeptide-
Intermediate Staphylococcus aureus Working Group. N Engl J Med
1999;340:493-501.
2. Fridkin SK. Vancomycin-intermediate and -resistant Staphylo-
coccus aureus: what the infectious disease specialist needs to know.
Clin Infect Dis 2001;32(1):108-15.
3. Flournoy DJ. Methicillin-resistant Staphylococcus aureus at a Vet-
erans Affairs Medical Center (1986-96). J Okla State Med Assoc
1997;90(6):228-35.
4. Istre GR, Tarpay M, Anderson M, Pryor A, Welch D. Pneumococcus
Study Group. Invasive disease due to Streptococcus pneumoniae in an
area with a high rate of relative penicillin resistance. J Infect Dis
1987;156:732-5.
5. Breiman RF, Spika JS, Navarro VJ, Darden PM, Darby CP. Pneu-
mococcal bacteremia in Charleston County, South Carolina: a decade
later. Arch Intern Med 1990;150:1401-5.
6. Stratton CW. Dead bugs don’t mutate: susceptibility issues in the
emergence of bacterial resistance. Emerg Infect Dis 2003;9(1):10-6.
7. Khan K, Muennig P, Behta M, Zivin JG. Global drug-resistance
patterns and the management of latent tuberculosis infection in immi-
grants to the United States. N Engl J Med 2002;347(23):1850-9.
8. Musoke RN, Revathi G. Emergence of multidrug-resistant gram-
negative organisms in a neonatal unit and the therapeutic implica-
tions. J Trop Pediatr 2000;46(2):86-91.
9. Mylonakis E, Calderwood SB. Infective endocarditis in adults.
N Engl J Med 2001;345(18):1318-30.
10. Doern GV, Ferraro MJ, Brueggemann AB, Ruoff KL. Emergence
of high rates of antimicrobial resistance among viridans group strepto-
cocci in the United States. Antimicrob Agents Chemother 1996;40:
891-4.
11. Jorgensen MG, Slots J. The ins and outs of periodontal antimicro-
bial therapy. J Calif Dent Assoc 2002;30(4):297-305.
12. Normark BH, Normark S. Evolution and spread of antibiotic
resistance. J Inter Med 2002;252(2):91-106.
13. Kozlova EV, Pivovarenko TV, Malinovskaia IV, Aminov RI,
Kovalenko NK, Voronin AM. Antibiotic resistance of Lactobacillus
strains [in Russian]. Antibiot Khimioter 1992;37(6):12-5.
14. Dajani AS, Taubert KA, Wilson W, et al. Prevention of bacterial
endocarditis: recommendations by the American Heart Association.
JADA 1997;128:1142-51.
15. American Dental Association; American Academy of Orthopedic
Surgeons. Antibiotic prophylaxis for dental patients with total joint
replacements. JADA 2003;134:895-9.
16. Herrera D, Sanz M, Jepsen S, Needleman I, Roldan S. A system-
atic review on the effect of systemic antimicrobials as an adjunct to
scaling and root planing in periodontitis patients. J Clin Periodontol
2002;29(supplement 3):136-59.
17. Ciancio SG, ed. ADA guide to dental therapeutics. 3rd ed.
Chicago: ADA Publishing; 2003:136-72.
18. Physicians’ desk reference. 58th ed. Montvale, N.J.: Medical
Economics; 2004:1321.
Copyright ©2004 American Dental Association. All rights reserved.
14
AAE Quick Reference Guide on Antibiotic Prophylaxis 2017 Update | Page 1
AAE Quick Reference Guide
Endocarditis Prophylaxis Recommendations
These recommendations are taken from 2017 American Heart Association and
American College of Cardiology focused update of the 2014 AHA/ADA Guideline
for Management of Patients with Valvular Disease (1) and cited by the ADA (2).
Prophylaxis against infective endocarditis is reasonable before dental
procedures that involve manipulation of gingival tissue, manipulation of the
periapical region of teeth, or perforation of the oral mucosa in patients with the
following:
In 2017, the AHA and American College of Cardiology (ACC) published
a focused update (5) to their previous guidelines on the management of
valvular heart disease. This reinforced their previous recommendations that
AP is reasonable for the subset of patients at increased risk of developing
IE and at high risk of experiencing adverse outcomes from IE (5). Their key
recommendations were:
1. Prosthetic cardiac valves, including transcatheter-implanted
prostheses and homografts.
2. Prosthetic material used for cardiac valve repair, such as
annuloplasty rings and chords.
3. Previous IE.
4. Unrepaired cyanotic congenital heart disease or repaired congenital
heart disease, with residual shunts or valvular regurgitation at the
site of or adjacent to the site of a prosthetic patch or prosthetic
device.
5. Cardiac transplant with valve regurgitation due to a structurally
abnormal valve.
Distribution Information
AAE members may reprint
this position statement for
distribution to patients or
referring dentists.
Antibiotic Prophylaxis
2017 Update
The guidance in this
statement is not intended
to substitute for a clinician’s
independent judgment in
light of the conditions and
needs of a specific patient.
About This Document
This paper is designed to
provide scientifically based
guidance to clinicians
regarding the use of antibiotics
in endodontic treatment.
Thank you to the Special
Committee on Antibiotic Use in
Endodontics: Ashraf F. Fouad,
Chair, B. Ellen Byrne, Anibal R.
Diogenes, Christine M. Sedgley
and Bruce Y. Cha.
©2017
Reprinted with permission from the
Am
erican Association of Endodontists.
Access additional resources at www.aae.org
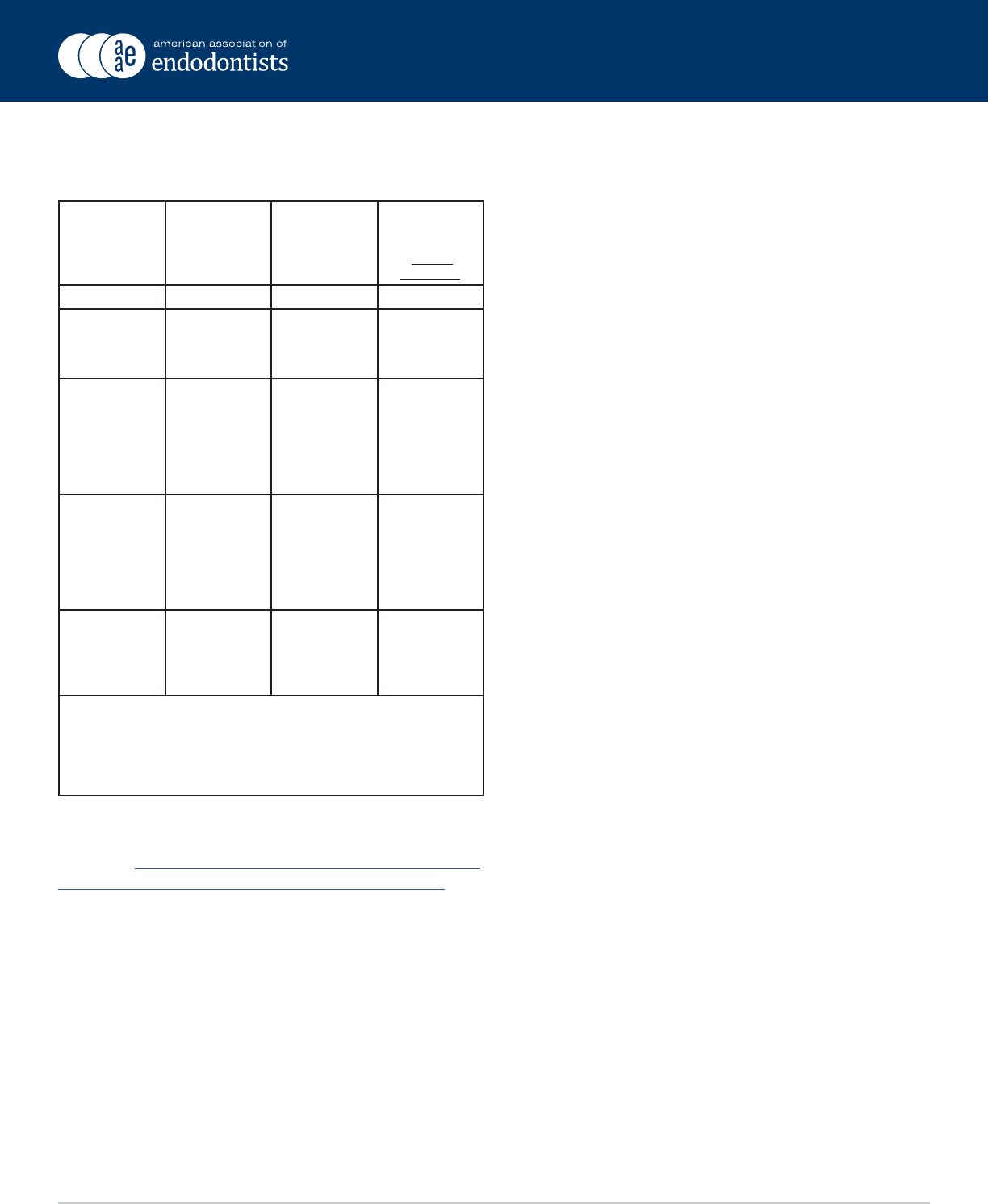
In 2017, the ADA reaffirmed the recommended regimen as
follows.
Regimen:
Single Dose 30
to 60 min.
Before
Procedure
Situation Agent Adults Children
Oral Amoxicillin 2 g 50 mg/kg
Unable to take
oral medication
Ampicillin
OR
Cefazolin or
ceftriaxone
2 g IM* or IV+
1 g IM or IV
50 mg/kg IM
or IV
50 mg/kg IM
or IV
Allergic to
penicillins or
ampicillin—oral
OR
Clindamycin
OR
Azithromycin or
clarithromycin
2 g
600 mg
500 mg
50 mg/kg
20 mg/kg
15 mg/kg
Allergic to
penicillins or
ampicillin and
unable to take
oral medication
Cefazolin or
OR
Clindamycin
1 g IM or IV
600 mg IM or IV
50 mg/kg IM
or IV
20 mg/kg IM
or IV
*IM: Intramuscular
+IV: Intravenous
adult or pediatric dosage.
anaphylaxis, angioedeme, or urticaria with penecillins or ampicillin.
The ADA and AHA have a downloadable wallet card available
to providers at no cost to educate patients who may be at
risk for IC. http://www.heart.org/idc/groups/heart-public/@
wcm/@hcm/documents/downloadable/ucm_448472.pdf
Patients with Join Replacement
The following recommendation is taken from the ADA
Chairside Guide (© ADA 2015)
• In general, for patients with prosthetic joint implants,
prophylactic antibiotics are not recommended prior to
dental procedures to prevent prosthetic joint infection.
• In cases where antibiotics are deemed necessary, it is most
appropriate that the orthopedic surgeon recommend the
appropriate antibiotic regimen and when reasonable write
the prescription
Additional Considerations
The practitioner and patient should consider possible
clinical circumstances that may suggest the presence of a
significant medical risk in providing dental care without
or widespread antibiotic use. As part of the evidence-based
approach to care, this clinical recommendation should be
integrated with the practitioner’s professional judgment in
consultation with the patient’s physician, and the patient’s
needs and preferences.
• These considerations include, but are not limited to:
• Patients with previous late artificial joint infection
• Increased morbidity associated with joint surgery (wound
drainage/hematoma)
• Patients undergoing treatment of severe and spreading
oral infections (cellulitis)
• Patient with increased susceptibility for systemic infection
•
• Patients on immunosuppressive medications
• Diabetics with poor glycemic control
• Patients with systemic immunocompromising disorders
(e.g. rheumatoid arthritis, lupus erythematosus)
• Patient in whom extensive and invasive procedures are
planned
• Prior to surgical procedures in patients at a significant risk
for medication-related osteonecrosis of the jaw.
Special Circumstances
The 2007 AHA guidelines state that an antibiotic for
prophylaxis should be administered in a single dose before
the procedure (3,4). However, in the event that the dosage
of antibiotic is inadvertently not administered before the
procedure, it may be administered up to two hours after the
procedure. For patients already receiving an antibiotic that
is also recommended for IE prophylaxis, then a drug should
be selected from a different class; for example, a patient
already taking oral penicillin for other purposes may likely
have in their oral cavity viridans group streptococci that are
relatively resistant to beta-lactams.
16

AAE Quick Reference Guide on Antibiotic Prophylaxis 2017 Update | Page 3
In these situations, clindamycin, azithromycin or
clarithromycin would be recommended for AP. Alternatively
if possible, treatment should be delayed until at least 10 days
after completion of antibiotic to allow re-establishment of
usual oral flora. In situations where patients are receiving
long-term parenteral antibiotic for IE, the treatment should
be timed to occur 30 to 60 min after delivery of the parenteral
antibiotic; it is considered that parenteral antimicrobial
therapy is administered in such high doses that the high
concentration would overcome any possible low-level
resistance developed among oral flora (3,4).
APPENDIX C REFERENCES
1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin
JP, 3rd, Fleisher LA, et al. 2017 AHA/ACC Focused Update
of the 2014 AHA/ACC Guideline for the Management
of Patients With Valvular Heart Disease: A Report of
the American College of Cardiology/American Heart
Association Task Force on Clinical Practice Guidelines.
Circulation 2017
2. ADA. Antibiotic prophylaxis prior to dental procedures.
Oral Health Topics 2017 [cited 31st March 2017];
Available from: http://www.ada.org/en/member-center/
oral-health-topics/antibiotic-prophylaxis
3. Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour
LM, Levison M, et al. Prevention of infective endocarditis:
guidelines from the American Heart Association: a
guideline from the American Heart Association Rheumatic
Fever, Endocarditis, and Kawasaki Disease Committee,
Council on Cardiovascular Disease in the Young, and the
Council on Clinical Cardiology, Council on Cardiovascular
Surgery and Anesthesia, and the Quality of Care and
Outcomes Research Interdisciplinary Working Group.
Circulation 2007;116:1736-54.
4. Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour
LM, Levison M, et al. Prevention of infective endocarditis:
guidelines from the American Heart Association: a
guideline from the American Heart Association Rheumatic
Fever, Endocarditis and Kawasaki Disease Committee,
Council on Cardiovascular Disease in the Young, and the
Council on Clinical Cardiology, Council on Cardiovascular
Surgery and Anesthesia, and the Quality of Care and
Outcomes Research Interdisciplinary Working Group. J
Am Dent Assoc 2008;139 Suppl:3S-24S.
17

AMERICAN ACADEMY OF PEDIATRIC DENTISTRY
RECOMMENDATIONS: BEST PRACTICES 371
Purpose
e American Academy of Pediatric Dentistry (AAPD) recog-
nizes the increasing prevalence of antibiotic-resistant micro-
organisms. is guideline is intended to provide guidance in
the proper and judicious use of antibiotic therapy in the
treatment of oral conditions.
1
Methods
is guideline was originally developed by the Council on
Clinical Aairs and adopted in 2001. is document is a
revision of the previous version, last revised in 2009. e re-
vision was based upon a new systematic literature search of
the PubMed
®
/MEDLINE database using the terms: antibiotic
therapy, antibacterial agents, antimicrobial agents, dental trau-
ma, oral wound management, orofacial infections, periodontal
disease, viral disease, and oral contraception; elds: all; limits:
within the last 10 years, humans, English, clinical trials, birth
through age 18. One hundred sixty-ve articles matched these
criteria. Papers for review were chosen from this search and
from hand searching. When data did not appear sucient or
were inconclusive, recommendations were based upon expert
and/or consensus opinion by experienced researchers and
clinicians.
Background
Antibiotics are benecial in patient care when prescribed and
administered correctly for bacterial infections. However, the
widespread use of antibiotics has permitted common bacteria
to develop resistance to drugs that once controlled them.
1-3
Drug resistance is prevalent throughout the world.
3
Some
microorganisms may develop resistance to a single anti-
microbial agent, while others develop multidrug-resistant
strains.
2,3
To diminish the rate at which resistance is increas-
ing, health care providers must be prudent in the use of
antibiotics.
1
Recommendations
Conservative use of antibiotics is indicated to minimize the
risk of developing resistance to current antibiotic regimens.
2,3
Practitioners should adhere to the following general princi-
ples when prescribing antibiotics for the pediatric population.
Oral wound management
Factors related to host risk (e.g., age, systemic illness, malnu-
trition) and type of wound (e.g., laceration, puncture) must
be evaluated when determining the risk for infection and
subsequent need for antibiotics. Wounds can be classied as
clean, potentially contaminated, or contaminated/dirty. Facial
lacerations may require topical antibiotic agents.
4
Intraoral
lacerations that appear to have been contaminated by extrinsic
bacteria, open fractures, and joint injury have an increased risk
of infection and should be covered with antibiotics.
4
If it is
determined that antibiotics would be benecial to the healing
process, the timing of the administration of antibiotics is
critical to supplement the natural host resistance in bacterial
killing. e drug should be administered as soon as possible for
the best result. e most eective route of drug administration
(intravenous vs. intramuscular vs. oral) must be considered.
e clinical eectiveness of the drug must be monitored. e
minimal duration of drug therapy should be ve days beyond
the point of substantial improvement or resolution of signs
and symptoms; this is usually a five- to seven-day course of
treatment dependent upon the specific drug selected.
5-7
In
light of the growing problem of drug resistance, the clinician
should consider altering or discontinuing antibiotics following
determination of either ineectiveness or cure prior to com-
pletion of a full course of therapy.
8
If the infection is not respon-
sive to the initial drug selection, a culture and susceptibility
testing of isolates from the infective site may be indicated.
Special conditions
Pulpitis/apical periodontitis/draining sinus tract/localized intra-
oral swelling
Bacteria can gain access to the pulpal tissue through caries,
exposed pulp or dentinal tubules, cracks into the dentin, and
defective restorations. If a child presents with acute symptoms
of pulpitis, treatment (i.e., pulpotomy, pulpectomy, or extrac-
tion) should be rendered. Antibiotic therapy usually is not
indicated if the dental infection is contained within the pulpal
tissue or the immediate surrounding tissue. In this case, the
Review Council
Council on Clinical Affairs
Latest Revision
2014
Use of Antibiotic Therapy for
Pediatric Dental
Patients
ABBREVIATION
AAPD: American Academy Pediatric Dentistry.
18
Copyright © 2017-18 by the American Academy of Pediatric Dentistry and reproduced with their permission.
372 RECOMMENDATIONS: BEST PRACTICES
REFERENCE MANUAL V 39
/
NO 6 17
/
18
child will have no systemic signs of an infection (i.e., no fever
and no facial swelling).
9,10
Consideration for use of antibiotics should be given in
cases of advanced non-odontogenic bacterial infections such
as staphylococcal mucositis, tuberculosis, gonococcal stoma-
titis, and oral syphilis. If suspected, it is best to refer patients
for culture, biopsy, or other laboratory tests for documentation
and denitive treatment.
Acute facial swelling of dental origin
A child presenting with a facial swelling or facial cellulitis sec-
ondary to an odontogenic infection should receive prompt
dental attention. In most situations, immediate surgical inter-
vention is appropriate and contributes to a more rapid cure.
12
e clinician should consider age, the ability to obtain adequate
anesthesia (local vs. general), the severity of the infection, the
medical status, and any social issues of the child.
11,12
Signs
of systemic involvement (i.e., fever, asymmetry, facial swelling)
warrant emergency treatment. Intravenous antibiotic therapy
and/or referral for medical management may be indicated.
9-11
Penicillin remains the empirical choice for odontogenic
infections; however, consideration of additional adjunctive
antimicrobial therapy (i.e., metronidazole) can be given where
there is anaerobic bacterial involvement.
8
Dental trauma
Systemic antibiotics have been recommended as adjunc-
tive therapy for avulsed permanent incisors with an open or
closed apex.
14-17
Tetracycline (doxycycline twice daily for seven
days) is the drug of choice, but consideration of the child’s
age must be exercised in the systemic use of tetracycline due
to the risk of discoloration in the developing permanent
dentition.
13,14
Penicillin V or amoxicillin can be given as an
alternative.
14,15,17
e use of topical antibiotics to induce pulpal
revascularization in immature non-vital traumatized teeth
has shown some potential.
14,15,17,18
However, further random-
ized clinical trials are needed.
19-21
For luxation injuries in the
primary dentition, antibiotics generally are not indicated.
22,23
Antibiotics can be warranted in cases of concomitant soft
tissue injuries (see Oral wound management) and when
dictated by the patient’s medical status.
Pediatric periodontal diseases
Dental plaque-induced gingivitis does not require antibiotic
therapy. Pediatric patients with aggressive periodontal diseases
may require adjunctive antimicrobial therapy in conjunction
with localized treatment.
24
In pediatric periodontal diseases
associated with systemic disease (e.g., severe congenital neutro-
penia, Papillon-Lefèvre syndrome, leukocyte adhesion de-
ciency), the immune system is unable to control the growth
of periodontal pathogens and, in some cases, treatment may
involve antibiotic therapy.
24,25
e use of systemic antibiotics
has been recommended as adjunctive treatment to mechanical
debridement in patients with aggressive periodontal disease.
24,25
In severe and refractory cases, extraction is indicated.
24,25
Cul-
ture and susceptibility testing of isolates from the involved
sites are helpful in guiding the drug selection.
24,25
Viral diseases
Conditions of viral origin such as acute primary herpetic gin-
givostomatitis should not be treated with antibiotic therapy
unless there is strong evidence to indicate that a secondary
bacterial infection exists.
26
Salivary gland infections
Many salivary gland infections, following conrmation of
bacterial etiology, will respond favorable to antibiotic therapy.
Acute bacterial parotitis has two forms: hospital acquired and
community acquired.
27
Both can be treated with antibiotics.
Hospital acquired usually requires intravenous antibiotics; oral
antibiotics are appropriate for community acquired. Chronic
recurrent juvenile parotitis generally occurs prior to puberty.
Antibiotic therapy is recommended and has been successful.
27
For both acute bacterial submandibular sialadenitis and chro-
nic recurrent submandibular sialadenitis, antibiotic therapy is
included as part of the treatment.
27
Oral contraceptive use
Whenever an antibiotic is prescribed to a female patient
taking oral contraceptives to prevent pregnancy, the patient
must be advised to use additional techniques of birth control
during antibiotic therapy and for at least one week beyond the
last dose, as the antibiotic may render the oral contraceptive
ineective.
28,29
Rifampicin has been documented to decrease
the eectiveness of oral contraceptives.
28,29
Other antibiotics,
particularly tetracycline and penicillin derivatives, have been
shown to cause signicant decrease in the plasma concentra-
tions of ethinyl estradiol, causing ovulation in some individuals
taking oral contraceptives.
28,29
Caution is advised with the
concomitant use of antibiotics and oral contraceptives.
28,29
References
1. Wilson W, Taubert KA, Gevitz M, et al. Prevention of in-
fective endocarditis: Guidelines from the American Heart
Association—A Guideline From the American Heart As-
sociation Rheumatic Fever, Endocarditis and Kawasaki
Disease Committee, Council on Cardiovascular Disease
in the Young, and the Council on Clinical Cardiology,
Council on Cardiovascular Surgery and Anesthesia Anes-
thesia, and the Quality of Care and Outcomes Research
Interdisciplinary Working Group. Circulation 2007;
116(15):1736-54. E-published April 19, 2007. Erratum
in: Circulation 2007;116(15):e376-e7.
2. Center for Disease Control and Prevention. Antibiotic/
Antimicrobial Resistance. Available at: “http://www.
cdc.gov/drugresistance/”. Accessed August 5, 2014.
3. Costelloe C, Metcalfe C, Lovering A, et al. Effect of
antibiotic prescribing in primary care on antimicrobial
resistance in individual patients: Systematic review and
meta-analysis. BMJ 2010;340:c2096.
19
AMERICAN ACADEMY OF PEDIATRIC DENTISTRY
RECOMMENDATIONS: BEST PRACTICES 373
4. Nakamura Y, Daya M. Use of appropriate antimicro-
bials in wound management. Emerg Med Clin North
Am 2007;25(1):159-76.
5. Wickersham RM, Novak KK, Schweain SL, et al. Sys-
temic anti-infectives. In: Drug Facts and Comparisons.
St. Louis, Mo.: 2004:1217-336.
6. Kuriyama T, Karasawa T, Nakagawa K, Saiki Y, Yamamoto
E, Nakamura S. Bacteriological features and antimicro-
bial susceptibility in isolates from orofacial odontogenic
infections. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod 2000;90(5):600-8.
7. Prieto-Prieto J, Calvo A. Microbiological basis of oral
infections and sensitivity to antibiotics. Med Oral Patol
Oral Cir Bucal 2004;9(suppl S):11-8.
8. Flynn T. What are the antibiotics of choice for odonto-
genic infections, and how long should the treatment
course last? Oral Maxillofac Surg Clin N Am 2011;23
(4):519-36.
9. Maestre Vera Jr. Treatment options in odontogenic infec-
tion. Med Oral Patol Oral Cir Bucal 2004;9(suppl S):
19-31.
10. Keenan JV, Farman AG, Fedorowicz Z, Newton JT. A
Cochrane system review nds no evidence to support the
use of antibiotics for pain relief in irreversible pulpitis.
J Endod 2006;32(2):87-92.
11. ikkurissy S, Rawlins JT, Kumar A, Evans E, Casamas-
simo PS. Rapid treatment reduces hospitalization for
pediatric patients with odontogenic-based cellulitis. Am J
Emerg Med 2010;28(6):668-672.
12. Johri A, Piecuch JF. Should teeth be extracted immedia-
tely in the presence of acute infection? Oral Maxillofac
Surg Clin North Am 2011;23(4):507-11.
13. Rega AJ, Aziz SR, Ziccardi VB. Microbiology and Anti-
biotic Sensitivities of Head and Neck Space Infections of
Odontogentic Origin. J Oral Maxillofac Surg 2006;64
(9):1377-1380.
14. Andreasen JO, Andreasen FM. Avulsions. In: Textbook
and Color Atlas of Traumatic Injuries to the Teeth, 4th
ed. Copenhagen, Denmark: Blackwell Munksgaard;
2007:461, 478-88.
15. Dentaltraumaguide.org. e Dental Trauma Guide 2010.
Permanent Avulsion Treatment. Available at “http://
www.dentaltraumaguide.org/Permanent_Avulsion_
Treatment.aspx”. Accessed October 1, 2013.
16. DiAngelis AJ, Andreasen JO, Ebelseder KA, et al. In-
ternational Association of Dental Traumatology Guide-
lines for the management of traumatic dental injuries:
1 – Fractures and luxations of permanent teeth. Dent
Traumatol 2012;28:2-12.
17. Andersson L, Andreasen JO, Day P, et al. International
Association of Dental Traumatology Guidelines for the
management of traumatic dental injuries: 2 – Avulsion
of permanent teeth. Dent Traumatol 2012;28:88-96.
18. McIntyre JD, Lee JY, Tropte M, Vann WF Jr. Manage-
ment of avulsed permanent incisors: A comprehensive
update. Pediatr Dent 2007;29(1):56-63.
19. Hargreaves KM, Diogenes A, Teixeira FB. Treatment
options: Biological basis of regenerative endodontic pro-
cedures. Pediatr Dent 2013;35(2):129-40.
20. ibodeau B, Teixeira F, Yamauchi M, Caplan DJ, Trope
M. Pulp revascularization of immature dog teeth with
apcial periodontitis. J Endod 2007;36(6):680-9.
21. Shabahang S. Treatment options: Apexogenesis and
apexication. Pediatr Dent 2013;35(2):125-8.
22. Malmgren B, Andreasen JO, Flores MT, et al. Interna-
tional Association of Dental Traumatology Guidelines for
the management of traumatic dental injuries: III. Injuries
in the primary dentition. Dental Traumatology 2012;
28:174-82.
23. Dentaltraumaguide.org. e Dental Trauma Guide: Pri-
mary Teeth, 2010. Available at “http://www.dental
traumaguide.org/Primary_teeth.aspx.” Accessed October
1, 2013.
24. American Academy of Periodontology Research, Science
and erapy Committee. Periodontal diseases of children
and adolescents. J Periodontol 2003;74:1696-704.
25. Schmidt JC, Wlater C, Rischewski JR, Weiger R. Treat-
ment of periodontitis as a manifestation of neutropenia
with or without systemic antibiotics: A systematic review.
Pediatr Dent 2013;35(2):E54-E63.
26. American Academy of Pediatrics. Herpes simplex. In: Red
Book: 2003 Report of the Committee on Infectious Dis-
eases. 26th ed. Elk Grove Village, Ill: American Academy
of Pediatrics; 2003:344-53.
27. Carlson ER. Diagnosis and management of salivary gland
infections. Oral Maxillofac Surg Clin N Am 2009;21
(3):293-312.
28. DeRossi SS, Hersh EV. Antibiotics and oral contracep-
tives. Pediatr Clin North Am 2002;46(4):653-64.
29. Becker DE. Adverse drug interactions. Anesth Prog 2011;
58(1):31-41.
20
Management of patients with prosthetic joints
undergoing dental procedures
Clinical Recommendation:
In general, for patients with prosthetic joint implants, prophylactic antibiotics are
not
recommended prior to dental procedures to prevent prosthetic joint infection.
For patients with a history of complications associated with their joint replacement surgery who are undergoing dental procedures
that include gingival manipulation or mucosal incision, prophylactic antibiotics should only be considered after consultation with
the patient and orthopedic surgeon.* To assess a patient’s medical status, a complete health history is always recommended when
making final decisions regarding the need for antibiotic prophylaxis.
Clinical Reasoning for the Recommendation:
• There is evidence that dental procedures are not associated with prosthetic joint implant infections.
• There is evidence that antibiotics provided before oral care do not prevent prosthetic joint implant infections.
• There are potential harms of antibiotics including risk for anaphylaxis, antibiotic resistance, and opportunistic infections
like Clostridium difficile.
• The benefits of antibiotic prophylaxis may not exceed the harms for most patients.
• The individual patient’s circumstances and preferences should be considered when deciding whether to prescribe prophylactic
antibiotics prior to dental procedures.
* In cases where antibiotics are deemed necessary, it is most appropriate that the orthopedic surgeon recommend the appropriate antibiotic regimen and when reasonable write the prescription.
Copyright © 2015 American Dental Association. All rights reserved. This page may be used, copied, and distributed
for non-commercial purposes without obtaining prior approval from the ADA. Any other use, copying, or distribution,
whether in printed or electronic format, is strictly prohibited without the prior written consent of the ADA.
Sollecito T, Abt E, Lockhart P, et al. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints: Evidence-based clinical practice guideline for dental practitioners — a report
of the American Dental Association Council on Scientific Affairs. JADA. 2015;146(1):11-16.
21
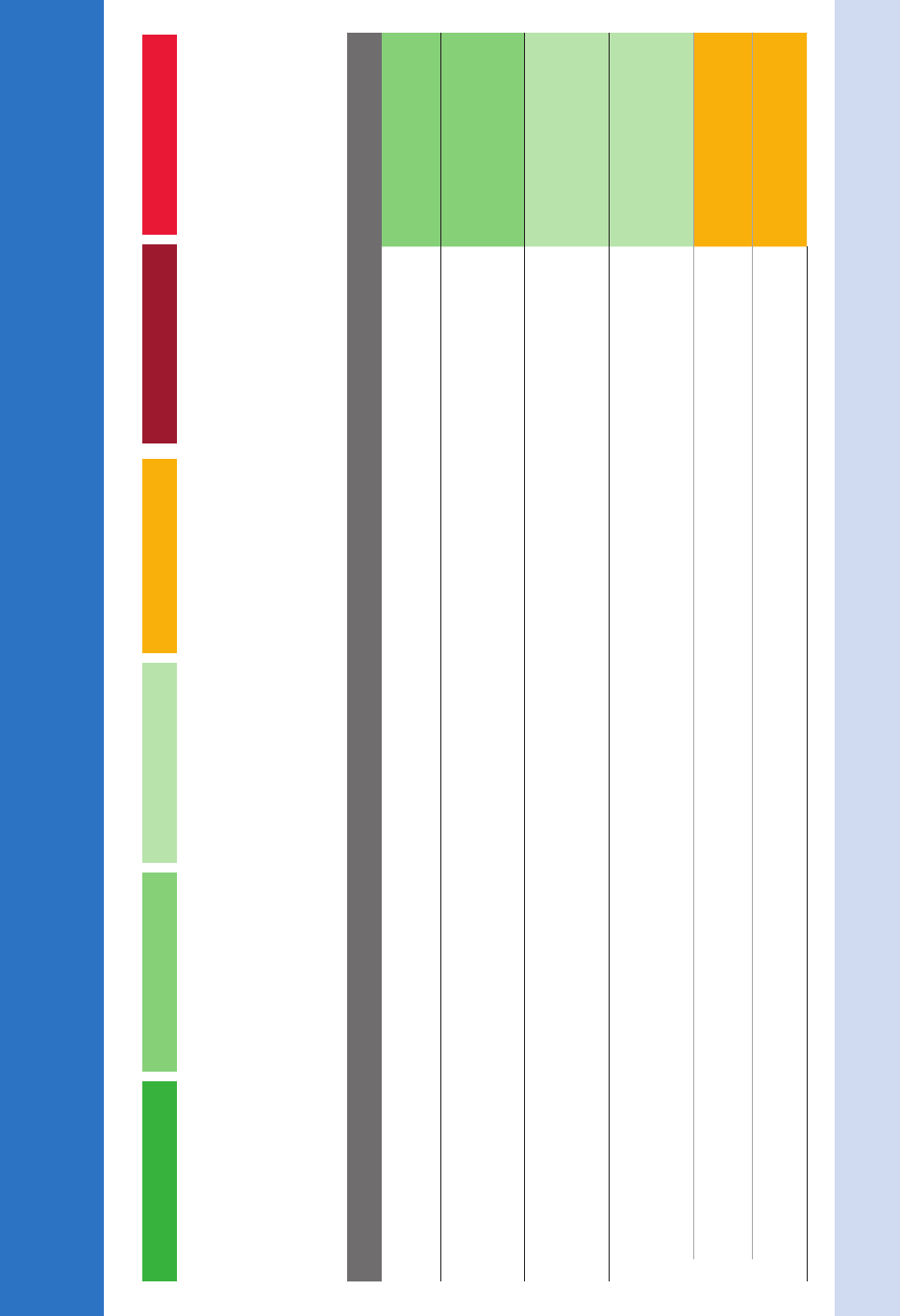
Nonsurgical Treatment of Chronic Periodontitis by Scaling and Root
Planing with or without Adjuncts: Clinical Practice Guideline
1,2
Strength of recommendations: Each recommendation is based on the best available evidence. The level of evidence available to support each recommendation may differ.
Evidence strongly supports
providing this intervention. There
is a high level of certainty of
benefits, and the benefits
outweigh the potential harms.
Strong
Expert Opinion suggests this
intervention can be implemented,
but there is a low level of certainty
of benefits and there is uncertainty
in the benefit to harm balance.
Expert Opinion For
Evidence favors providing this
intervention. Either there is a high
level of certainty of benefits, but
the benefits are balanced with
the potential harms OR there
is a moderate level of certainty
of benefits, and the benefits
outweigh the potential for harms.
In Favor
Expert Opinion suggests this
intervention NOT be implemented
because there is a low level of
certainty that there is no benefit
or the potential harms outweigh
benefits.
Expert Opinion Against
Evidence suggests implementing
this intervention only after alterna-
tives have been considered. There
is a moderate level of certainty of
benefits, and either the benefits
are balanced with potential harms
or there is uncertainty in the
magnitude of the benefit.
Weak
Evidence suggests not
implementing this intervention
or discontinuing ineffective
procedures. There is moderate
or high certainty that there are
no benefits and/or the potential
harms outweigh the benefits.
Against
Clinical Recommendation Strength
Scaling and root planing (no adjuncts)
For patients with chronic periodontitis, clinicians should consider scaling and root planing (SRP) as the initial treatment.
In Favor
SRP with systemic sub-antimicrobial dose doxycycline
For patients with moderate to severe chronic periodontitis, clinicians may consider systemic sub-antimicrobial dose doxycycline (20 mg twice a day) for
3 to 9 months as an adjunct to SRP with a small net benefit expected.
In Favor
SRP with systemic antimicrobials
For patients with moderate to severe chronic periodontitis, clinicians may consider systemic antimicrobials as an adjunct to SRP with a small net benefit
expected.
Weak
SRP with locally-delivered antimicrobials
For patients with moderate to severe chronic periodontitis, clinicians may consider locally delivered chlorhexidine chips as an adjunct to SRP with a
moderate net benefit expected.
Weak
For patients with moderate to severe chronic periodontitis, clinicians may consider locally delivered doxycycline hyclate gel as an adjunct to SRP, but
the net benefit is uncertain.
Expert Opinion For
For patients with moderate to severe chronic periodontitis, clinicians may consider locally delivered minocycline microspheres as an adjunct to SRP,
but the net benefit is uncertain.
Expert Opinion For
1 Smiley CJ, Tracy SL, Abt E, Michalowicz B, et al. Evidence-Based Clinical Practice Guideline on the Nonsurgical Treatment of Chronic Periodontitis by Scaling and Root Planing with or without Adjuncts. JADA 2015; 146 (7):525-535.
2 Smiley CJ, Tracy SL, Abt E, Michalowicz B, et al. Systematic Review and Meta-Analysis on the Nonsurgical Treatment of Chronic Periodontitis by Scaling and Root Planing with or without Adjuncts. JADA 2015; 146 (7):508-524.
©2015 American Dental Association. All rights reserved.
22
Copyright © 2015 American Dental Association. All rights reserved. Adapted with permission. To see full text of this article, please go to JADA/ADA.org/cgi/content/ This page may be used, copied, and distributed for
non-commercial purposes without obtaining prior approval from the ADA. Any other use, copying, or distribution, whether in printed or electronic format, is strictly prohibited without the prior written consent of the ADA.
Center for Evidence-Based Dentistry
™
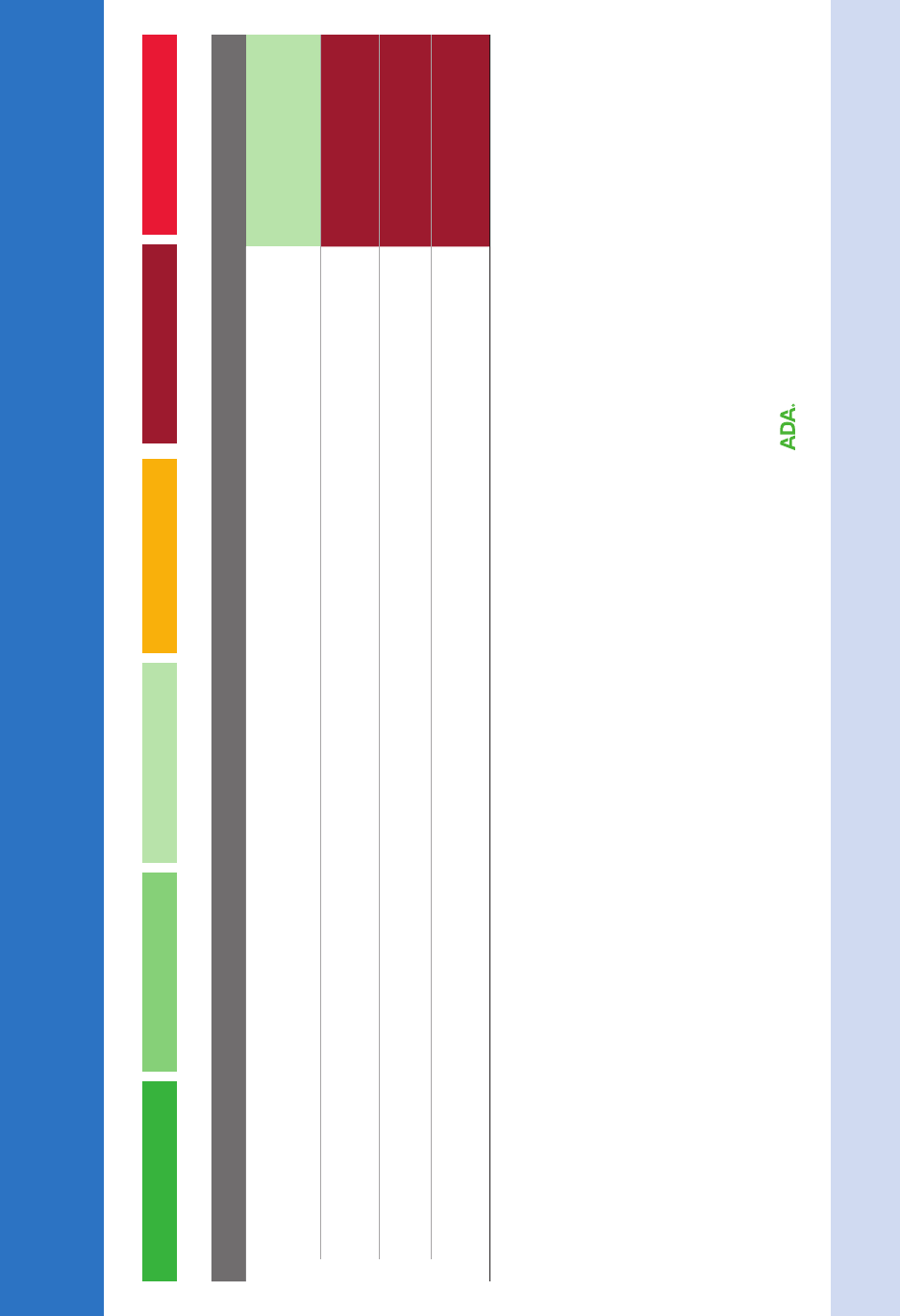
Nonsurgical Treatment of Chronic Periodontitis by Scaling and Root
Planing with or without Adjuncts: Clinical Practice Guideline
1,2
Clinical Recommendation Strength
SRP with nonsurgical use of lasers
For patients with moderate to severe chronic periodontitis, clinicians may consider photodynamic therapy (PDT) using diode lasers as an adjunct
to SRP with a moderate net benefit expected.
Weak
For patients with moderate to severe chronic periodontitis, clinicians should be aware that the current evidence shows no net benefit from diode
(non-PDT) lasers when used as an adjunct to SRP.
Expert Opinion Against
For patients with moderate to severe chronic periodontitis, clinicians should be aware that the current evidence shows no net benefit from Nd:YAG
lasers when used as an adjunct to SRP.
Expert Opinion Against
For patients with moderate to severe chronic periodontitis, clinicians should be aware that the current evidence shows no net benefit from erbium
lasers when used as an adjunct to SRP.
Expert Opinion Against
Strength of recommendations: Each recommendation is based on the best available evidence. The level of evidence available to support each recommendation may differ.
Strong Expert Opinion For In Favor Expert Opinion Against Weak Against
23

24
3. Track and Report
Tracking and reporting antibiotic prescribing can guide changes in practice and be
used to assess progress in improving antibiotic prescribing. Dentists can track and
report antibiotic prescribing practices by doing the following:
Complete the enclosed survey
This tool is intended to help you assess your facility’s current antibiotic prescribing
practices and identify areas for improvement. It will also help IDPH learn how to
support dentists’ antibiotic stewardship efforts. Please complete this brief survey
online at http://tinyurl.com/survey4dentists by December 22, 2017.
Rather complete the survey by mail? A postage-paid envelope has been included for
your convenience. You may also fax the completed survey to: 312-814-1953.
Participate in continuing medical education and quality improvement activities to track
and improve antibiotic prescribing
Attend the Annual Illinois Summit on Antimicrobial Stewardship next summer. This
annual event convenes clinicians across health care settings to discuss antibiotic
stewardship best practices. More information on the summit will be shared in spring
2018. To be added to the e-mail list, contact: [email protected]v.
Implement a tracking and reporting system in your facility to monitor antibiotic
prescribing
Refer to CDC’s resources on tracking at: https://tinyurl.com/CDCtrack.

25
4. Educate
Dentists can educate patients about the potential harms of antibiotic treatment with
the following tools:
Antibiotic Safety: Do’s & Don’ts at the Dentist (page 26)
Download here: http://tinyurl.com/patiented1.
What is antibiotic prophylaxis? (page 27)
Download here: http://tinyurl.com/patiented2.
What is Infective endocarditis? (page 28)
Download here: http://tinyurl.com/patiented3.
Improving Antibiotic Use (page 30)
Download here: http://tinyurl.com/patiented4.

Antibiotic Safety: Do’s and Don’ts at the Dentist
DO
9 DO tell your dentist if you
have any drug allergies or
medical conditions.
9 DO tell your dentist about
any medications, vitamins, or
herbal supplements you are
taking.
9 DO ask how some mouth
infection can be treated
without antibiotics.
9 DO take your antibiotics
exactly as prescribed.
9 DO tell your dentist if you
have side effects, such as
frequent diarrhea, while
taking, or shortly after
stopping antibiotics.
DO NOT
X DO NOT skip doses or stop
taking your antibiotics
without consulting your
dentist.
X DO NOT save unused
antibiotics for future use or
give antibiotics to others.
X DO NOT take antibiotics
prescribed for others.
X DO NOT pressure your
dentist to prescribe an
antibiotic. Instead, ask your
dentist how you can feel
better even if antibiotics are
not prescribed.
CS267104
https://www.cdc.gov/getsmart/community/downloads/dos-and-donts---final.pdf
26

What is antibiotic
prophylaxis?
A
ntibiotics usually are used to treat bacterial
infections. Sometimes, though, dentists or
physicians suggest taking antibiotics before
treatment to decrease the chance of infection.
This is called antibiotic prophylaxis.
During some dental treatments, bacteria from the
mouth enter the bloodstream. In most people, the im-
mune system kills these bacteria. There is concern,
though, that in some patients, bacteria from the mouth
can travel through the bloodstre am and cause an infec-
tion somewhere else in the body. Antibiotic prophylaxis
may offer these people extra protection.
1
WHO MIGHT BENEFIT FROM ANTIBIOTIC
PROPHYLAXIS?
People with certain heart conditions may be at increased
risk of developing infective endocarditis (IE)—an infec-
tion of the lining of the heart or heart valves. To protect
against IE, or limit its effects should the infection
develop, the American Heart Association suggests that
antibiotic prophylaxis be considered for people who
have
1
-
an artificial heart valve or who have had a heart valve
repaired with a prosthetic material;
-
a history of IE;
-
a heart transplant that develops a valve problem;
-
certain heart conditions that are congenital (present
from birth), including
n unrepaired or incompletely repaired cyanotic
congenital heart disease, including those with palliative
shunts and conduits;
n a completely repaired congenital heart defect with
prosthetic material or device, whether placed by surgery
or by catheter intervention, during the first 6 months
after the procedure;
n any repaired congenital heart disease with residual
defect at the site or adjacent to the site of a prosthetic
patch or prosthetic device.
WHAT ABOUT PEOPLE WHO HAVE HAD HIP OR KNEE
REPLACEMENT SURGERY?
The American Dental Association does not routinely
recommend antibiotic prophylaxis for people who have
had a hip, knee, or other joint replaced.
2
People who
have had joint replacement surgery and have a weakened
immune system—meaning that they are less able to fight
infections—should talk to their dentist and their ortho-
pedic surgeon to see if antibiotic prophylaxis is recom-
mended. Conditions such as diabetes, rheumatoid
arthritis, or cancer and medications such as steroid s
and those used in chemotherapy can affect your ability to
fight infections.
WHY IS ANTIBIOTIC PROPHYLAXIS NOT USED FOR
EVERY PATIENT?
Antibiotic prophylaxis is not right for everyone and—
like any medicine—antibiotics should only be used when
the potential benefits outweigh the risks of taking them.
For example, consider that infections after dental treat-
ment are not common and that, in some people, anti-
biotics can have side effects. Side effects associated with
taking antibiotics include stomach upset, diarrhea, and
allergic reactions, some of which can be life threatening.
In addition, using antibiotics too often or incorrectly can
allow bacteria to become resistant to those medications.
Therefore, it is important to use antibiotic prophylaxis in
only those people at greatest risk of developing an
infection after dental treatment.
WHAT CAN YOU DO?
Tell your dentist about any changes in your health since
your last visit and make sure he or she knows about all
medications you are taking. With this information in
hand, your dentist can talk to you and your physician
about whether you could benefit from antibiotic
prophylaxis.
Good home care is key to good dental health. Be
sure to brush you r teeth twice a day with a fluoride
toothpaste, clean between your teeth once a day, eat
a balanced diet, and visit your dentist regularly.
n
http://dx.doi.org/10.1016/j.adaj.2016.03.016
Prepared by Anita M. Mark, manager, Scientific Information Develop-
ment, ADA Science Institute, American Dental Association, Chicago, IL.
Disclosure. Ms. Mark did not report any disclosures.
Copyright Ó 2016 American Dental Association. Unlike other portions of
JADA, the print and online versions of this page may be reproduced as a
handout for patients without reprint permission from the ADA Publishing
Division. Any other use, copying, or distribution of this material, whether in
printed or electronic form, including the copying and posting of this ma-
terial on a website is prohibited without prior written consent of the ADA
Publishing Division.
“For the Patient” provides general information on dental treatments. It is
designed to prompt discussion between dentist and patient about treatment
options and does not substitute for the dentist’s professional assessment
based on the individual patient’s needs and desires.
1. Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective
endocarditis: guidelines from the American Heart Association: a guideline
from the American Heart Association Rheumatic Fever, Endocarditis and
Kawasaki Disease Committee, Council on Cardiovascular Disease in the
Young, and the Council on Clinical Cardiology, Council on Cardiovascular
Surgery and Anesthesia, and the Quality of Care and Outcomes Research
Interdisciplinary Working Group. JADA. 2008;139(suppl):3S-24S.
2. Sollecito TP, Abt E, Lockhart PB, et al. The use of prophylactic anti-
biotics prior to dental procedures in patients with prosthetic joints:
evidence-based clinical practice guideline for dental practitioners—a report
of the American Dental Association Council on Scientific Affairs. JADA.
2015;146(1):11-16.e8.
FOR THE PATIENT
526 JADA 147(6) http://jada.ada.org June 2016
27
(continued)
ANSWERS
by
heart
What’s the role of bacteria?
Certain bacteria normally live on parts of your body.
They live in or on the:
• mouth and upper respiratory system.
• intestinal and urinary tracts.
• skin.
Bacteria can get in the bloodstream. This is called
bacteremia. These bacteria can settle on abnormal,
damaged, or prosthetic heart valves or other damaged
heart tissue. If this happens, they can damage or even
destroy the heart valves.
The heart valves are important in guiding blood ow
through the heart. They work like doors to keep the
blood owing in one direction. If they become damaged,
the results can be very serious.
A brief bacteremia can occur after many routine daily
activities such as:
• tooth brushing and ossing.
• use of wooden toothpicks.
• use of water picks.
• chewing food.
It can also result after certain surgical and dental
procedures. Not all bacteria cause endocarditis, though.
What’s the heart’s role?
People who have certain heart conditions are at
increased risk of developing infective endocarditis.
People with the highest risk for poor outcomes from
IE may be prescribed antiobiotics prior to dental
procedures to reduce their risk of developing IE.
Heart conditions that put people at the highest risk for
poor outcomes from IE include:
• articial (prosthetic) heart valves or heart valves
repaired with articial material
• a history of infective endocarditis
• some kinds of congenital heart defects
• abnormality of the heart valves after a heart transplant
People who’ve had IE before are at higher risk of getting
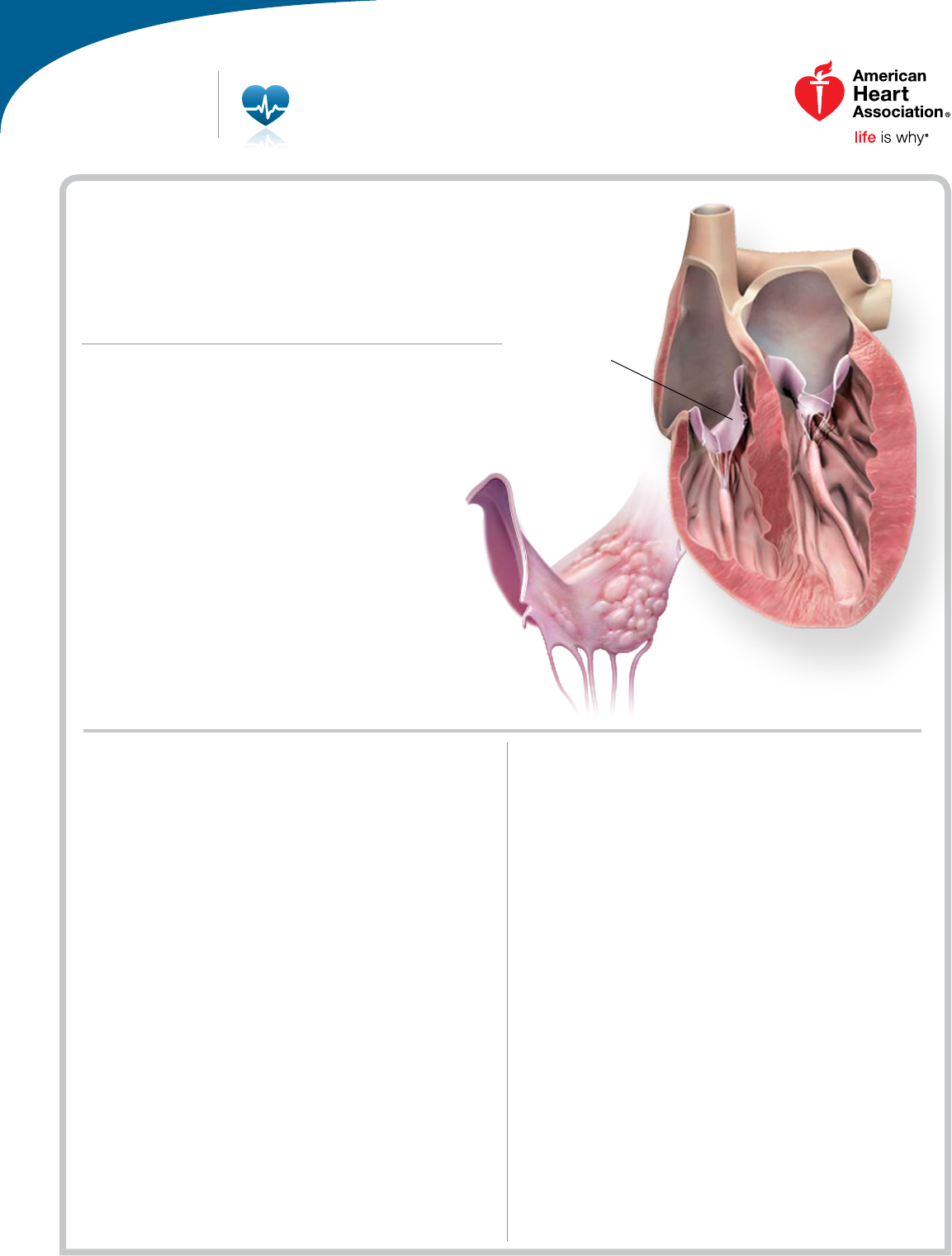
Infective (bacterial) endocarditis (IE) is an
infection of either the heart’s inner lining
(endocardium) or the heart valves. Infective
endocarditis is a serious — and sometimes
fatal — illness. Two things increase risk for
it to occur: pathogens such as bacteria
or fungi in the blood and certain high-risk
heart conditions.
Men, women and children of all racial
and ethnic groups can get it. In the
United States, there are up to 34,000
hospital discharges r
elated to IE each year.
What Is Infective
Endocarditis?
Cardiovascular Conditions
Below: closeup
of a tricuspid
valve damaged
by infective
endocarditis
Healthy
tricuspid
valve
28

Cardiovascular Conditions
ANSWERS
by
heart
What Is Infective Endocarditis?
ANSWERS
by
heart
it again. This is true even when they don’t have heart
disease.
How can infective endocarditis be
prevented?
Not all cases can be prevented. That’s because we don’t
always know when an infection will occur.
For patients whose heart conditions put them at the
highest risk for adverse events from IE, the American
Heart Association (AHA) recommends antibiotics before
certain dental procedures. These include procedures that
involve manipulation of gingival tissue or the periapical
region of teeth, or perforation of the oral mucosa.
However, for most patients, antibiotics are not needed.
The AHA has an endocarditis wallet card in English
and Spanish. People who have been told that they need
to take antibiotics should carry it. You can get it from
your doctor or on our Web site,
heart.org. Show the card
to your dentist or physician. It will help them take the
precautions needed to protect your health.
Keeping your mouth clean and healthy and maintaining
regular dental care may reduce the chance of bacteremia
from routine daily activities.
Take a few minutes to
write your questions for
the next time you see
your healthcare provider.
For example:
Call 1-800-AHA-USA1
(1-800-242-8721), or visit heart.org
to learn more about heart disease and
stroke.
Sign up to get Heart Insight, a free
magazine for heart patients and their
families, at heartinsight.org.
Connect with others sharing similar
journeys with heart disease and stroke
by joining our Support Network at
heart.org/supportnetwork.
We have many other fact sheets to help you make healthier choices to reduce your risk,
manage disease or care for a loved one. Visit heart.org/answersbyheart to learn more.
HOW CAN I LEARN MORE?
Do you have
questions for the
doctor or nurse?
My Questions:
©2017, American Heart Association
What conditions do I
have that put me at risk
for endocarditis?
Should I take antibiotics
before I see the dentist?
Patients whose heart conditions put them at risk for IE may reduce
the risk by practicing good dental hygiene. In some cases, they may
need to take antibiotics prior to dental procedures.
29
IMPROVING
ANTIBIOTIC USE
Do I really need antibiotics?
SAY YES TO ANTIBIOTICS
when needed for certain infections caused
by
bacteria.
SAY NO TO ANTIBIOTICS
for viruses, such as colds and flu, or runny
noses, even if the mucus is thick, yellow or
green. Antibiotics also won’t help for some
common bacterial infections including most
cases of bronchitis, many sinus infections,
and some ear infections.
Antibiotics do NOT work on
viruses.
Antibiotics
are only
needed for
treating
certain
infections
caused by
bacteria.
Do antibiotics have side effects?
Anytime antibiotics are used, they can cause side effects. When antibiotics aren’t
needed, they won’t help you, and the side effects could still hurt you. Common side
effects of antibiotics can include:
More serious side eects include Clostridium dicile infection
(also called C. dicile or C. di), which causes diarrhea that can
lead to severe colon damage and death. People can also have
severe and life-threatening allergic reactions.
Antibiotics save lives. When a patient needs
antibiotics, the benefits outweigh the risks
of side effects.
Rash Yeast Infections
Dizziness
Nausea
Diarrhea
1 out of 5
medication-related
visits to the ED are from
reactions to antibiotics.
30
What are antibiotic-resistant bacteria?
Antibiotic resistance occurs when bacteria no longer respond to the
drugs designed to kill them. Anytime antibiotics are used, they can cause
antibiotic resistance.
Bacteria, not the
body, become
resistant to the
antibiotics designed
to kill them.
When bacteria
become resistant,
antibiotics cannot
fight them, and the
bacteria multiply.
Some resistant
bacteria can be
harder to treat
and can spread
to other people.
Each year in the
United States, at
least
2 million
people get
infected with
antibiotic-resistant
bacteria. At
least
23,000
people
die
as a result.
Can I feel better without antibiotics?
Respiratory viruses usually go away in a week or two without treatment. To
stay healthy and keep others healthy, you can:
Clean Hands
Cover Coughs
Stay Home
When Sick
Get
Recommended
Vaccines
To learn more about antibiotic prescribing and use,
visit www.cdc.gov/antibiotic-use.
31
REFERENCES
Abt, E., Hellstein, J., Lockhart, P., Mariotti, A., Sollecito, T., Truelove, E., Armstrong, S., De
Rossi, S., Epstein, J., Laudenbach, J., Patton, L., Paumier, T. and Weyant, R. (2017).
American Dental Association guidance for utilizing appropriate use criteria in the
management of the care of patients with orthopedic implants undergoing dental
procedures. The Journal of the American Dental Association, 148(2), pp.57-59.
American De
ntal Association. (2017). Antibiotics and Dental Treatment brochure - ADA W307.
[online] Available at: http://ebusiness.ada.org/productcatalog/205/
Medication/Antibiotics-and-Dental-Treatment/W307
.
CDC.gov. (2017). Measuring Outpatient Antibiotic Prescribing. [online] Available at:
https://www.cdc.gov/antibiotic-use/community/programs-measurement/measuring-
antibiotic-prescribing.html
CDC.gov
. (2017). Print Materials for Healthcare Professionals. [online] Available at:
https://www.cdc.gov/antibiotic-use/community/materials-references/print-
materials/hcp/index.html.
Combatin
g antibiotic resistance. (2004). The Journal of the American Dental Association,
135(4), pp.484-487.
Guideline o
n Use of Antibiotic Therapy for Pediatric Dental Patients. (2014). American Academy
of Pediatric Dentistry Clinical Practice Guidelines, 37(6), pp.289-291.
Roberts
, R., Bartoces, M., Thompson, S. and Hicks, L. (2017). Antibiotic prescribing by general
dentists in the United States, 2013. The Journal of the American Dental Association,
148(3), pp.172-178.e1.
Wilson,
W., Taubert, K., Gewitz, M., Lockhart, P., Baddour, L., Levison, M., Bolger, A., Cabell,
C., Takahashi, M., Baltimore, R., Newburger, J., Strom, B., Tani, L., Gerber, M., Bonow,
R., Pallasch, T., Shulman, S., Rowley, A., Burns, J., Ferrieri, P., Gardner, T., Goff, D.
and Durack, D. (2007). Prevention of Infective Endocarditis: Guidelines From the
American Heart Association: A Guideline From the American Heart Association
Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on
Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council
on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes
Research Interdisciplinary Working Group. Circulation, 116(15), pp.1736-1754.
32